This article is an installment of Future Explored, a weekly guide to world-changing technology. You can get stories like this one straight to your inbox every Thursday morning by subscribing here.
Northwestern University researchers have found that they can supercharge cancer vaccines simply by structuring their ingredients in a precise way — and if the discovery translates from mice to people, it could forever change how we design vaccines.
“The collective importance of this work is that it lays the foundation for developing the most effective forms of vaccine for almost any type of cancer,” said study author Michelle Teplensky. “It is about redefining how we develop vaccines across the board, including ones for infectious diseases.”
The challenge: Vaccines for harmful pathogens, like coronavirus, work by training the immune system to recognize a specific germ before a person is sick. If it ever infects them in the future, their immune system can jump into action before an infection runs wild.
The part of the vaccine that triggers an immune response is called the “antigen.” It might be a harmless bit of the target pathogen (a bit of protein), or a weakened or killed version of the whole thing. The mRNA vaccines contain genetic instructions that teach the body to make antigens.
“But there must be an optimum ratio of each that would maximize the vaccine’s effectiveness.”
Michelle Teplensky
Cancer vaccines typically work a bit differently.
Rather than trying to prevent disease, they try to treat it by triggering the immune system to identify and attack cancerous cells, which slip under its radar. The antigens for these vaccines tend to be one or more specific molecules found on tumor cells.
Whether a vaccine is designed to target cancer or pathogens, many include a substance that boosts the immune response. This is called an “adjuvant,” and it can be anything from aluminum salts to nucleic acids.
Typically, the adjuvant and the antigen are simply mixed together during manufacturing.
“A challenge with conventional vaccines is that out of that blended mish mosh, an immune cell might pick up 50 antigens and one adjuvant or one antigen and 50 adjuvants,” said Teplensky. “But there must be an optimum ratio of each that would maximize the vaccine’s effectiveness.”
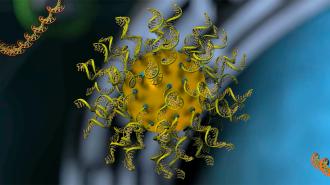
Rational vaccinology: In 1996, a team led by Chad Mirkin, director of Northwestern’s International Institute for Nanotechnology (IIN), invented a structure consisting of a nanoparticle core covered in strands of nucleic acids, giving it the appearance of a “koosh ball.”
They called it a “spherical nucleic acid” (SNA), and it has since been used in clinical trials to deliver drugs into cells, with promising results.
In 2019, the team demonstrated how SNAs could be used as a modular platform for cancer vaccines, too — tumor antigens and nucleic acid adjuvants could be loaded up together, and the nanostructures would deliver them to immune cells.
This approach, which they dubbed “rational vaccinology,” allows developers to specify how much of each vaccine ingredient to be delivered into each cell.
“Vaccines developed through rational vaccinology deliver the precise dose of antigen and adjuvant to every immune cell, so they are all equally primed to attack cancer cells,” said Mirkin.
“If your immune cells are soldiers, a traditional vaccine leaves some unarmed; our vaccine arms them all with a powerful weapon with which to kill cancer,” he continued. “Which immune cell ‘soldiers’ do you want to attack your cancer cells?”
Rational vaccinology also gives developers the ability to dictate the precise locations of vaccine ingredients on the SNAs — the antigens could be inside the nanoparticle, attached to its surface, or attached to the ends of the nucleic acids, like in the image below.
The 2019 study revealed that this has a surprising effect on efficacy — depending on the structure, the same vaccine ingredients could be ineffective or capable of curing treated animals’ cancer.
What’s new? Most cancer vaccines are designed to activate one type of immune cell: killer T cells. For their latest study, the Northwestern team demonstrated how an SNA vaccine, loaded with multiple antigens, can activate helper T cells, too.
“The more types of cells the immune system has to go after tumors, the better,” said Teplensky. “Vaccines consisting of multiple antigens targeting multiple immune cell types are necessary to induce enhanced and long-lasting tumor remission.”
Compared to an SNA cancer vaccine with just one antigen, the multiple-antigen vaccine triggered the production of twice as many antigen-specific T cells and increased their activation by 30%, in a mouse model of aggressive melanoma.
The researchers also discovered that they could increase the efficacy of the multi-antigen vaccine by tweaking its structure.
“It is remarkable,” Mirkin said. “When altering the placement of antigens in two vaccines that are nearly identical from a compositional standpoint, the treatment benefit against tumors is dramatically changed. One vaccine is potent and useful, while the other is much less effective.”
“The developments made in this work provide a path forward to rethinking the design of vaccines for cancer and other diseases as a whole,” he added.
Looking ahead: Mirkin’s team believes rational vaccinology could increase the potency of many types of vaccines — in 2022, they demonstrated a vaccine for COVID-19 that protected 100% of mice from a lethal dose of the coronavirus.
It might not be long before we start finding out whether the promising results of these mouse studies translate to people — Flashpoint Therapeutics, a startup founded by Mirkin, hopes to begin clinical trials of an SNA-based vaccine for cervical cancer in 2024.
“We can’t tell you that [rational vaccinology] will always lead to curative vaccines, but we can tell you it will lead to better vaccines,” Mirkin told Reuters.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at tips@freethink.com.