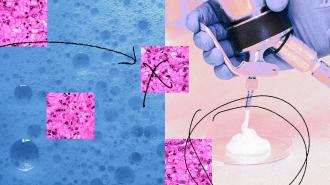
A drinkable foam packed with carbon monoxide molecules appears to boost the effects of autophagy inhibitors, an experimental cancer therapy that previously had yielded mixed results in patients.
The background: Cells are complex little machines, and after so long, different parts within them wear out or become damaged. Through a process called “autophagy,” cells get rid of those useless parts or break them down and repurpose pieces of them.
This process is vital to cells’ survival, and unfortunately, cancer cells are particularly adept at it. This gave researchers the idea that drugs that block autophagy might be effective cancer therapies, but trials of autophagy inhibitors in cancer patients have been inconclusive.
“Within those clinical trials, they found mixed results; there was some benefit, but for many patients there was no benefit, which really pushed researchers back to the drawing board,” said James Byrne, an assistant professor of radiation oncology at the University of Iowa (UI).
“We saw an increase in overall response in smokers that received the autophagy inhibitors.”
James Byrne
The idea: To figure out why autophagy inhibitors are so inconsistent as a cancer therapy, Byrne and his colleagues took a look back at completed trials. That’s when they discovered the bizarre result, in two small studies, that patients who were still actively smoking actually responded better to the drugs than did non-smokers.
“[W]e saw an increase in overall response in smokers that received the autophagy inhibitors, compared to (non-smoker) patients, and we also saw a pretty robust decrease in the target lesion size,” said Byrne.
Smoking increases carbon monoxide (CO) levels in the body, and past research has shown that CO increases cellular autophagy. Previous studies have also suggested that deliberately inducing autophagy in cancer patients while administering inhibitors can make the drugs more effective — if more cancer cells need autophagy to survive, inhibitors can lead to more of them dying.

This led the researchers to hypothesize that smoking was boosting autophagy in patients in a way that made inhibitors more effective.
Telling cancer patients to smoke to increase the chance a therapy will work is, obviously, not a viable option — smoking causes cancer, among other things. So the researchers looked for a different, theoretically safer way to increase CO levels in the body temporarily — and landed on a drinkable foam packed with CO molecules.
The study: To test the foam, the UI team fed it to mouse models of pancreatic and prostate cancer while treating them with autophagy inhibitors. Compared to controls given just the drugs, tumor growth and progression was significantly reduced in the foam-fed animals.
“The results from this study support the idea that safe, therapeutic levels of CO … can increase the anti-cancer activity of autophagy inhibitors.”
James Byrne
Tests of autophagy inhibitors on human prostate, lung, and pancreatic cancer cells in the lab revealed that the drugs had a stronger anti-cancer effect when paired with the foam, as well.
The CO foam by itself, meanwhile, appeared to have no effect on the viability of healthy or cancerous human cells in testing, and doses large enough to be therapeutic when combined with autophagy inhibitors appeared safe and tolerable when tested in mice and pigs.
Looking ahead: This is still early research, but the UI team hopes to test its combination therapy in future trials, and the fact that two autophagy inhibitors (chloroquine and hydroxychloroquine) are already approved by the FDA to treat other health issues could streamline their path to the clinic — if they work.
“The results from this study support the idea that safe, therapeutic levels of CO, which we can deliver using [gas-entrapping materials], can increase the anti-cancer activity of autophagy inhibitors, opening a promising new approach that might improve therapies for many different cancers,” said Byrne.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].