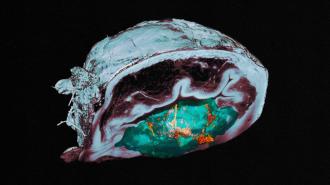
Tiny nanobots propelled by urine and carrying radioactive particles shrank bladder tumors by 90% in mice — a promising development in the fight against one of the world’s most difficult cancers.
The challenge: Bladder cancer is the tenth most common type of cancer in the world. Thankfully, it’s also one of the easiest to catch early — about 75% of cases are diagnosed before the tumor has had a chance to grow into the bladder’s muscle layer.
While the standard treatment for bladder tumors at this stage is generally effective at preventing death, it isn’t great at preventing them from coming back — nearly half of bladder cancer patients experience a recurrence within 5 years, and the need for repeated screenings and treatments makes bladder cancer one of the most expensive types of cancer to have.
Bladder cancer is the tenth most common type of cancer in the world.
The status quo: Bladder cancer treatment typically begins with the removal of as much of the tumor as possible via surgery, followed by a second treatment called “intravesical therapy.”
A drug — usually chemo or immunotherapy — is inserted directly into the patient’s bladder via a catheter. They then wait an hour or two before using the restroom to give the therapy time to work. Depending on the drug used, side effects include fatigue, flu-like symptoms, frequent urination, and pain while urinating.
Intravesical therapy may be the only treatment administered for hard-to-remove bladder tumors, but whether used solo or in combination with surgery, a patient may need to repeat the therapy as frequently as once a week for several years, depending on their perceived risk of recurrence.
“Patients with this type of tumor typically have 6 to 14 hospital appointments with current treatments.”
Samuel Sánchez
What’s new? Making intravesical therapy more effective could help reduce bladder cancer recurrence rates, not to mention free patients from the cost and discomfort of frequent treatments.
Now, researchers in Spain have developed “nanobots” to deliver a radiopharmaceutical — a type of drug that uses radiation to kill cancer — through intravesical therapy. When injected into the bladders of mouse models of bladder cancer, the rodents’ tumors shrank by 90%.
“This is significantly more efficient given that patients with this type of tumor typically have 6 to 14 hospital appointments with current treatments,” said study leader Samuel Sánchez from the Institute for Bioengineering of Catalonia (IBEC). “Such a treatment approach would enhance efficiency, reducing the length of hospitalization and treatment costs.”
How it works: Each nanobot is a tiny sphere made of silica. Radioactive iodine is attached to its surface, along with urease, a protein that reacts with a compound found in urine. The reaction propels the nanobots, which helps them reach all parts of the bladder’s inner wall.
“This phenomenon favored greater tumor penetration.”
Meritxell Serra Casablancas
Using a combination of imaging techniques, the researchers saw that the nanobots tended to accumulate in the bladder tumors of their mice, which was a welcome surprise — there was no obvious reason for the nanobots to target those tissues.
With more research, though, they were able to figure out what was happening.
“[W]e observed that these nanorobots can break down the extracellular matrix of the tumor by locally increasing the pH through a self-propelling chemical reaction,” said co-first author Meritxell Serra Casablancas. “This phenomenon favored greater tumor penetration and was beneficial in achieving preferential accumulation in the tumor.”
The big picture: More research is needed to see whether bladder tumors treated with the nanobots are less likely to recur, and even if that’s the case in mice, it might not prove true in people if the treatment reaches clinical trials.
Radiopharmaceuticals are also challenging to manufacture, but interest in their use against cancer has been growing, and this study reveals a new way they could be used to treat one of the most financially burdensome cancers for patients.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].