While commonly seen as an unsightly impediment, fat, or adipose tissue, is actually integral to the proper functioning of the human body. Fat cells, called adipocytes, safely store excess nutrients as lipids, preventing these chemicals from aggregating in other tissues and clogging up the works, so to speak. For thousands of years, these lipid stores have served humanity as an on-demand energy source, built up in times of plenty and broken down for energy in times of scarcity. But passive energy storage isn’t all that body fat is good for.
“Adipose tissue regulates many aspects of whole-body physiology, including food intake, maintenance of energy levels, insulin sensitivity, body temperature, and immune responses,” a team of researchers from the University of Pennsylvania and the University of California-Los Angeles write in a new review paper published to the journal Cell.
These researchers, led by Claudio J. Villanueva at UCLA and Patrick Seale at Penn, argue that too much body fat isn’t actually what makes obese individuals unhealthy. The problem is that their fat tissue stops functioning normally.
The problem isn’t body fat itself
Body fat is an essential organ, and a remarkable one at that. Consider its unparalleled ability to expand and contract — no other organ can increase and decrease in size as body fat does. That’s because adipocytes can massively balloon as they suck up lipids like microscopic sponges, then deflate as they release them. At the same time, fat tissue secretes hormones called adipokines that regulate metabolism, affect satiety, and play a role in inflammation.
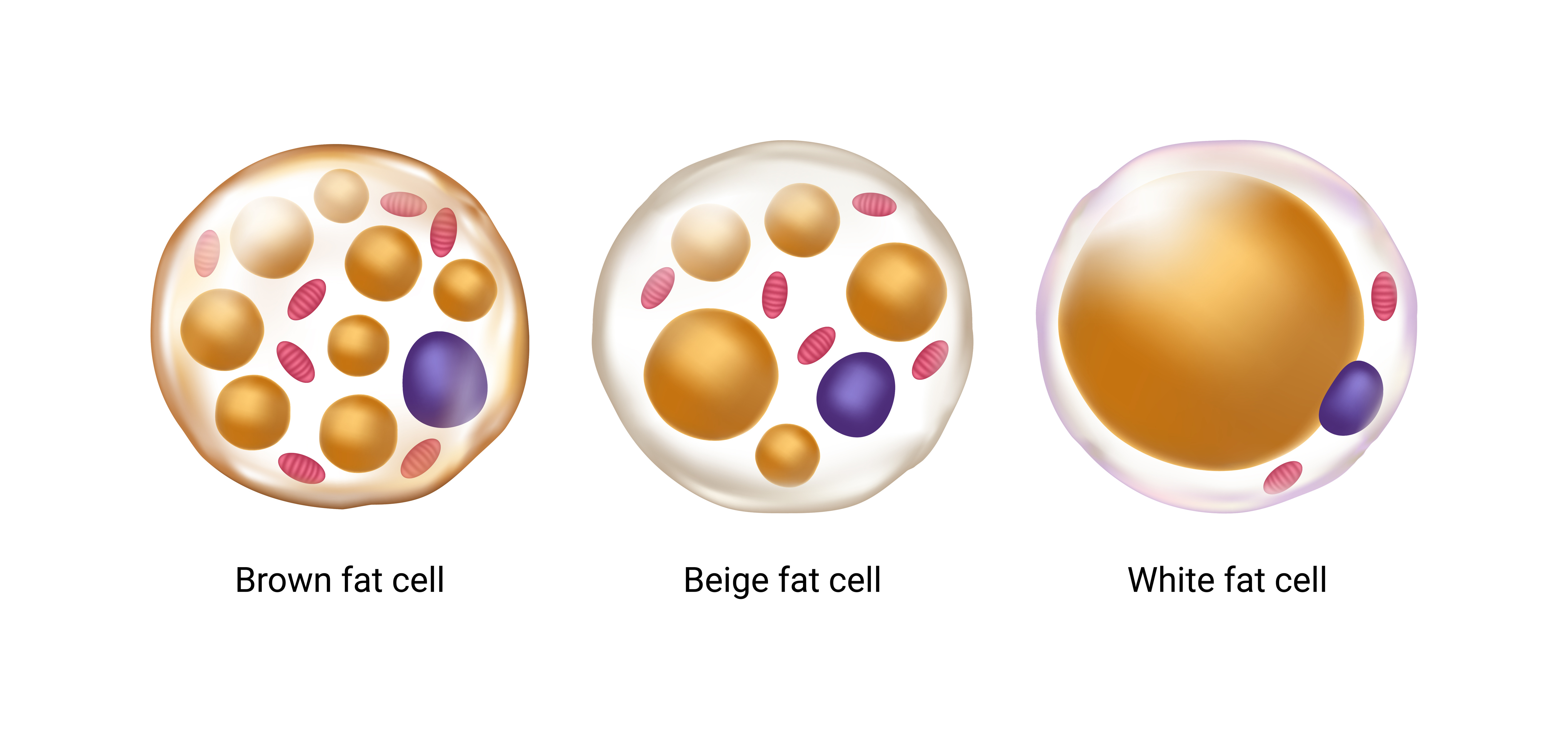
There are actually three types of body fat: white, brown, and beige. While white fat — the most common — is specialized solely for storage and hormonal function, beige and brown produce heat to keep us warm. Brown fat is stored in deposits whose locations are specified prior to birth (often around the collarbone and spine), while beige fat develops within stores of white fat. In fact, white fat cells actually convert to beige cells when environmental temperatures drop and revert back when temperatures rise.
This ability highlights one of the hallmarks of body fat: like brain tissue, adipose tissue is incredibly “plastic” — that is, it changes and adapts depending upon the body’s needs. Unfortunately, as Villanueva, Seale, and their colleagues point out, this plasticity can take a big hit in individuals with obesity.
When good fat goes bad
Citing a plethora of prior research, they suggest that some adipocytes can grow too large to receive enough oxygen from the available vasculature (the blood supply) causing these cells to die. This, in turn, leads to a cascade of detrimental effects: Dead fat cells cannot divide and create more cells, making it harder to store excess nutrients from food intake. Necrotizing adipocytes spill their own stored lipids, causing bodily havoc. Inflammatory cytokines are excreted. “Consequently, adipose tissue becomes insulin resistant, inflamed, and fibrotic, further compromising its function. All of these processes are continuous and mutually reinforcing, making it difficult to disentangle cause and effect,” the authors write.
This problem isn’t necessarily unique to individuals with obesity — even adipocytes in normal weight individuals can sometimes malfunction in this manner. But obese individuals do have more, often larger, fat cells, and thus more opportunities for their fat’s function to falter.
“Estimates from the United States suggest that 23.5% of normal-weight adults are metabolically unhealthy while 31.7% of obese are metabolically healthy,” the researchers write.
Metabolically unhealthy individuals often have high blood sugar, hypertension, and low HDL cholesterol, putting them at increased risk of stroke, heart failure, and a range of other diseases. The researchers suggest that drugs targeted to improve blood flow to fat cells could help resolve these issues.
“Many questions and opportunities for future discovery remain, which will yield new insights into adipose-tissue biology and hopefully lead to improved therapies for human disease.”
This article was reprinted with permission of Big Think, where it was originally published.