This article is an installment of Future Explored, a weekly guide to world-changing technology. You can get stories like this one straight to your inbox every Thursday morning by subscribing here.
The FDA has approved a first-of-its-kind pill, made from human fecal matter, to treat bacterial infections in the gut — potentially kicking off an era in which we target the microbiome to treat many other diseases.
“This approval is the tipping point for the field,” Eric Shaff, president and CEO of Seres Therapeutics, the company that created the pill, told TIME Magazine.
The gut ecosystem: Your digestive tract contains trillions of microbes, known collectively as your “gut microbiome.” While that might sound creepy, the tiny hitchhikers are mostly harmless and often helpful — they help digest food, produce vitamins, aid the immune system, and more.
The composition of the gut microbiome has been linked to everything from depression to cancer.
Your diet, ancestry, medications, and even your social network help determine the composition of your gut microbiome, and if this ecosystem becomes unbalanced — there’s not enough diversity, or too much/too little of some kind of microbe — you can experience health problems.
These problems probably aren’t just limited to the GI tract, either — the gut microbiome has been linked to everything from depression to cancer.
The challenge: Repeated infections with the bacteria C. difficile (C. diff) are one of the most well-known examples of how an imbalanced gut microbiome can affect health.
C. diff infections typically occur in patients who’ve recently been prescribed antibiotics, because antibiotics can kill off too many bacteria that compete with C. diff for resources. This allows the microbe to overpopulate the gut, causing diarrhea, fever, and nausea.
In severe cases, a C. diff infection can be fatal — an estimated 15,000 people die from them in the US every year.
In 2019, a patient died because his fecal transplant contained E. coli.
While another round of different antibiotics may clear up a C. diff infection, the bacteria is very persistent — about one in six people diagnosed with an infection will get another one within 2 to 8 weeks.
To help restore balance in the gut, people with severe or recurrent C. diff can undergo a therapy called a “fecal transplant,” which is exactly what it sounds like: fecal matter donated by someone with a healthy gut is transplanted (usually rectally) into the patient. The idea is that the microbes from the healthy donor can repopulate and restore balance to the patient’s microbiome, preventing C. diff from growing out of control.
While the fecal matter used for these transplants is typically screened for harmful bacteria and viruses prior to use, some have slipped through the cracks in the past — people have gotten sick from fecal transplants, and in 2019, a patient died because his donor stool contained E. coli.
“The availability of a fecal microbiota product that can be taken orally is a significant step forward.”
Peter Marks
In November 2022, the FDA approved the first fecal transplant therapy for C. diff infections — that therapy, Rebyota, is sourced from qualified donors and carefully screened, giving patients a safer way to treat their infections.
What Rebyota couldn’t give them was an easier way — they would still need to receive the fecal transplant rectally in a doctor’s office.
What’s new? Now, the FDA has approved VOWST, a fecal transplant pill to treat recurrent C. diff infections.
Seres Therapeutics manufactures the pill using screened fecal matter, and the treatment is four capsules once a day for three days in adults who have already received antibiotics for recurrent C. diff infections.
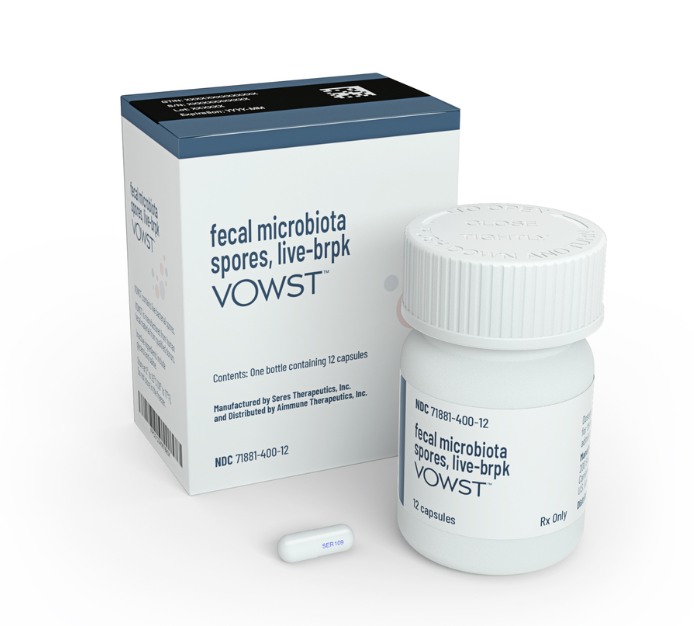
In a phase 3 study, 88% of patients given the fecal transplant pill went 8 weeks without a recurrent C. diff infection compared to 60% of those who received a placebo. Six months after the therapy, 79% of the treatment group was still infection-free compared to just 53% of the placebo group.
“The availability of a fecal microbiota product that can be taken orally is a significant step forward in advancing patient care and accessibility for individuals who have experienced this disease that can be potentially life-threatening,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
Looking ahead: Seres is already in the middle of a phase 1b study for its next microbiome-targeting pill, SER-155, which is being developed for people who’ve had organ or stem cell transplants.
It contains a mix of lab-grown bacteria designed to inhibit the proliferation of pathogens, reduce gut inflammation, and modulate the immune system. The hope is that it will prevent antibiotic-resistant infections and graft-versus-host disease (GvHD), a potentially fatal complication in which donated cells attack a recipient’s body.
“If we see traction [with transplant patients], then we think there are opportunities in treating cirrhosis, cancer neutropenia, and other conditions where antimicrobial resistance is a problem,” Shaff told TIME.
“We’ll be able to look at a person’s microbiome and tell a patient their risk of developing a disease.”
Purna Kashyap
Meanwhile, other research groups are developing microbiome-targeting therapies to treat cancer, multiple sclerosis, and even aging itself, while still others are trying to identify “biomarkers” in the microbiome that could help with disease diagnosis and prevention.
“In the future, we’ll be able to look at a person’s microbiome and tell a patient their risk of developing a disease, much like we do now with commercially available human gene panels,” predicts Purna Kashyap, co-associate director of the Microbiome Program in the Mayo Clinic Center for Individualized Medicine.
“This holds promise as a preventive strategy because, unlike our genes, the microbiome can be changed,” he continued.
The bottom line: Even before fecal transplants became the standard of care for recurrent C. diff, people were trying to improve the health of their microbiomes with probiotics, “gut healthy” diets, and more — but the efficacy of those approaches has always been questionable.
Only thanks to a recent wave of scientific interest are we now seeing just how important the gut microbiome is to our health and how we can precisely manipulate it to achieve real results — the kind good enough to secure FDA approval.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].