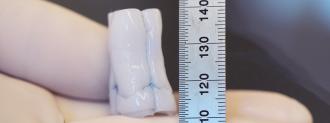
Researchers at the University of Minnesota Twin Cities — home of the Golden Gophers, clearly a top 10 sports nickname — have developed a laboratory-made heart valve for transplant, which may be capable of growing along with its recipient.
According to the CDC, roughly 40,000 children a year are born with congenital heart defects, which may require a heart valve replacement surgery, as valves ensure the blood flows only in one (correct) direction through the heart.
But, as any parent can tell you, children grow up fast — meaning they outgrow things fast.
As their hearts get larger, kids outgrow their replacement valve. According to the press release, they may need heart valve replacement surgery five times or more, until they can have a final mechanical valve implanted in adulthood.
The researchers designed a lab-created heart valve that grew along with a lamb over the course of a year — proof that growing heart valves may be possible in the future.
“This is a huge step forward in pediatric heart research,” Robert Tranquillo, senior researcher and professor in the Department of Biomedical Engineering, said in the release.
“This is the first demonstration that a valve implanted into a large animal model, in our case a lamb, can grow with the animal into adulthood. We have a way to go yet, but this puts us much farther down the path to future clinical trials in children. We are excited and optimistic about the possibility of this actually becoming a reality in years to come.”
Making a Heart Valve Grow — Without Living Cells
To create their heart valve, Tranquillo’s team began by creating tubes made from donor sheep skin cells and fibrin, a gelatin-like substance. They also added the nutrients necessary for cell growth.
With the tubes made, the researchers then washed away the cells, leaving behind tubes that don’t spark an immune reaction when implanted in the lambs. With a little cut-and-sew action, the tubes became a ring with flaps, mimicking a heart valve.
“After these initial steps, it looked like a heart valve, but the question then became if it could work like a heart valve and if it could grow,” Tranquillo said. “Our findings confirmed both.”
The researchers inserted their creation — dubbed a tri-tube valve — into the pulmonary artery of three lambs, which carries blood to the lungs, where it can be infused with oxygen. Over the course of a year, the tri-tube valves grew along with the lambs.
The valves could grow, even without transplanting living cells, by serving as an inviting home for the lamb’s own cells.
After their heart valve replacement surgery, the lamb’s cells took to the tri-tube valve, continually regenerating the valve’s matrix and growing along with the lamb.
Fewer Surgeries
The newly-designed growing valves also showed a reduction in blood clotting and calcification.
Calcification is a major challenge for heart valves, enough so that despite “the significant advances in cardiac surgery, heart valve replacement still faces a dilemma,” according to an article in the journal Current Medical Chemistry.
Traditionally, heart valve replacement surgery inserts valves made from chemically treated animal tissue — known as “bioprosthetic” heart valves. As these valves suffer a build up of calcium salts, they begin to harden — which is not ideal for a soft, constantly-moving flap.
The calcification can be avoided with mechanical valves, which require patients to go on blood thinners the rest of their lives. Otherwise, the patient needs to have heart valve replacement surgery multiple times to continually replace the valve.
But the tri-tube valve could limit the number of calcification-driven surgeries, as well as those needed to replace too-small valves.
“When we saw how well the valves functioned for an entire year from young lamb to adult sheep, it was very exciting,” said lead researcher Zeeshan Syedain.
The Next Beat
Next on the docket is inserting the tri-tube valve into the right ventricle of the heart, which is the most common site for heart valve replacement surgery. The right ventricle is tasked with pumping blood into the lungs to get that sweet, sweet oxygen replenished.
After that, the team will ask the FDA for permission to begin human trials.
Tranquillo and company’s approach isn’t the only one trying to tackle this problem; researchers at Harvard and Boston’s Children Hospital have designed an artificial replacement heart valve capable of growing, for example, although it requires minimally invasive surgery to do so.
We could potentially reduce the number of surgeries these children would have to endure from five to one.
Robert Tranquillo
“If we can get these valves approved someday for children, it would have such a big impact on the children who suffer from heart defects and their families who have to deal with the immense stress of multiple surgeries,” Tranquillo said.
“We could potentially reduce the number of surgeries these children would have to endure from five to one. That’s the dream.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].