A brain protein linked to memory formation could be the foundation of new Alzheimer’s treatments that don’t just slow disease progression but could actually reverse memory loss.
The treatment hasn’t yet been tried in people, so we don’t know if it’ll work in real Alzheimer’s cases, or if it would improve life expectancy for people with the disease.
The challenge is huge: There’s a lot we don’t understand about Alzheimer’s disease, but we do know that patient’s brains tend to accumulate toxic tau and amyloid-beta proteins, so most research has focused on those targets.
That approach has led to new drugs that can slow the progression of Alzheimer’s to a small degree, but we’ve yet to find anything that can reverse the damage the disease does to the brain.
“It was very surprising how strong the relationship was.”
Tara Tracy
The big idea: Synapses — the connections between brain neurons — need a protein called “KIBRA” in order to form memories, and there’s a link between certain variants of the KIBRA gene and developing Alzheimer’s.
This inspired scientists at the Buck Institute for Research on Aging to dig deeper into the protein to see whether it could lead to new Alzheimer’s treatments.
What’s new? For their study, the Buck team measured KIBRA levels in the cerebrospinal fluid (CSF) of 25 older adults (10 with Alzheimer’s and 15 who were clinically healthy), as well as in frozen brain tissue samples from people who died with and without Alzheimer’s.
They discovered a correlation between decreased KIBRA levels in the brain but elevated levels in CSF and how severe patients’ dementia was.
“We also found this amazing correlation between increased tau levels and increased KIBRA levels in the cerebrospinal fluid,” said senior author Tara Tracy. “It was very surprising how strong the relationship was, which really points to the role of KIBRA being affected by tau in the brain.”
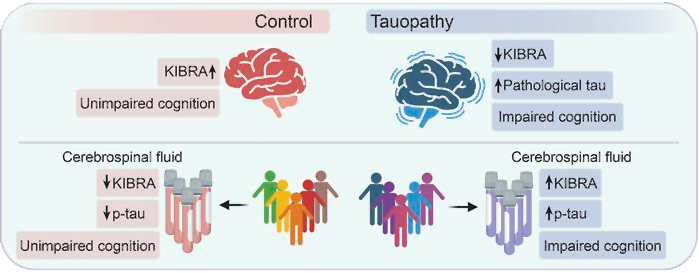
They aren’t sure why having more tau in the brain seems to lead to more KIBRA in CSF — in their study, they suggest that neurons with dysfunctional synapses might somehow expel the protein, causing it to accumulate in CSF, but confirming that would require more research.
Still, simply knowing about the connection could be useful — measuring KIBRA levels in a person’s CSF might one day help doctors diagnose Alzheimer’s or track its progression, for example.
A new treatment? The scientists injected a lab-made version of the KIBRA protein into the brains of mouse models of Alzheimer’s — and it reversed their memory loss.
“Interestingly, KIBRA restored synaptic function and memory in mice, despite not fixing the problem of toxic tau protein accumulation,” said co-first author Kristeen Pareja-Navarro.
In other words, the protein reversed a symptom (memory loss) even without apparently affecting the underlying disease.
Looking ahead: This is still early research, and results in mice often don’t translate to people — especially in Alzheimer’s research. Still, the Buck team is hopeful its study will prove to be a stepping stone to much needed new Alzheimer’s treatments.
“Reducing toxic proteins is of course important, but repairing synapses and improving their function is another critical factor that could help,” said Tracy. “That’s how I see this making the biggest impact in the future.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].