“Because 10 million people get TB every year,” Helen McShane says.
That, in short, is why the Oxford vaccinologist has spent her life creating vaccines for it.
Now, she has developed a new way to accelerate her life’s work: testing vaccines by purposefully trying to infect volunteers — an approach known as “human challenge trials.” Until recently, these experiments have been too dangerous to ethically consider running. Until the breakthrough.
1.5 Million. Every Year.
Though considered in most of the developed world to be relegated to historical dramas — ghostly Victorian ladies waning away, cowboys coughing blood into a bandanna before high noon — TB is not only a current threat to public health, but one whose disease burden and death toll was second to none, before COVID-19.
An estimated 1.5 to 1.6 million people — roughly the population of Philadelphia — die each year from tuberculosis. Each year.
TB is difficult to treat, requiring six months worth of antibiotics to do the job, and even longer for drug-resistant TB. The bacteria is infectious, especially in crowded conditions like cities and multi-generational homes; it hits hardest in low- and middle-income countries, which account for over 80% of all reported cases and deaths.
Tuberculosis is not only a current threat to public health, but one whose disease burden and death toll was second to none before COVID-19.
“TB’s a ‘poor man’s disease,’” McShane says, making it unattractive for pharmaceutical development because there’s little money in it, on top of being hard to kill or prevent. A high-risk, low-reward project. (Nevermind that someone could contract extensively drug-resistant TB in, say, South Africa, and be wheels-down at London’s Heathrow Airport within half a day, as she tells her medical students.)
There is a vaccine for TB, named Bacille Calmette-Guérin (BCG) after its French creators, which has been used for over a century and given to billions. But it protects infants from TB infection in the brain, not adults against the lung infection that is most common and most contagious.
So despite 85% of the world’s population having received it, McShane says, “BCG does not work well enough.”
To create a better vaccine and potentially save over a million lives per year, researchers need to “de-risk” vaccine development for pharmaceutical companies. One way to do so is through human challenge trials (HCTs).
Vaccinating and then purposefully infecting volunteers gives you rapid answers, which means finding out if your new vaccine or drug works faster (and cheaper) than having to run gigantic trials, involving potentially tens of thousands of people.
But, as previously mentioned, TB is a potentially deadly and persistent infection. That makes deliberately exposing people to it — even if they are young, healthy volunteers and receive proper care — ethically fraught territory.
However, there may be an alternative — it may have been staring us in the face for a century.
The All-Consuming
Symptoms of TB infection in the lungs include fever, night sweats, chronic coughing and spitting of blood, severe weight loss, and pallor — hence its designation as “the white death.” This wasting away led to its other popular name, “consumption,” and TB — despite its long, deadly history — became a symbol of beauty and art in the 19th century, a fashionable disease.
The bacteria itself, Mycobacterium tuberculosis, is weakly gram-positive, rod-shaped, and has numerous tricks that make it hard to fight. This includes a number of genes that help it evade the immune system and protect itself from drugs, along with a waxy coating that’s tough to penetrate. It has ways to distract and dampen the immune system around it.
This gives it the ability to lie low in its host for extended periods of time — potentially years — without causing disease. (In fact, billions of people are silently harboring some of these bacteria, without it necessarily leading to TB.)
“Because of all of those things, and because of its size and complexity, it’s a tough target,” says Corey Casper, a clinical professor of global health at the University of Washington and president and CEO of the Access to Advanced Health Institute (AAHI).
An estimated 1.5 to 1.6 million people — roughly the population of Philadelphia — die each year from tuberculosis. A vaccine which protects against TB in the lungs could save hundreds of thousands of lives annually.
The Challenge
There are two main reasons why using real or “wildtype” TB in a human challenge trial would be unethical, McShane says.
First, the treatment for TB is long, lasting at least six months, and the therapy can be toxic, especially to the liver. And, at the end of treatment, there is no way to test if the patient is truly cured, McShane says. After being purposefully infected, treated, and sent on their way, lingering latent TB may return in full force later in that patient’s life. (This, to McShane, is “the real killer” for the idea of using the bacteria as is.)
That is a considerable problem, because an HCT for TB could be an especially effective tool for vaccine development.
Because TB may stay hidden in people for a long time and is only detectable once it causes disease, it’s difficult to see if a potential vaccine has worked unless you look at a very large number of people over a very long time, says Harvard’s Sarah Fortune — and that’s very expensive.
Fortune, the chair of the Harvard TH Chan School of Public Health’s Department of Immunology and Infectious Diseases, leads a center for TB research, including developing ways to safely do human challenges.
The biggest benefit of an HCT would be making vaccine development faster and less risky. Take 20 people, give them your vaccine, infect them, and you could see if it works a month. Compare that to a phase 3 trial, which could involve thousands of people over years. The HCT is cheaper, faster, and makes the costly investment needed to deliver an approved vaccine less risky, because companies know up front whether it’s likely to work.
A key requirement for an HCT is having enough people willing to volunteer. In communities where TB is a clear and present threat, like Malawi, volunteers are ready, willing, and organizing, says Zacharia Kafuko. Kafuko is the director of 1Day Sooner Africa, a nonprofit that advocates for HCTs and for respecting the role of volunteers as stakeholders in science.
“The overwhelming perception from the community is very positive,” Kafuko says. “People there are actually happy and ready to be involved in a TB study.”
Another requirement: a safe, ethical way for scientists to run such a trial.
A human challenge trial could help TB vaccine development, but TB treatment is long and taxing, and there is no way to ensure a patient is truly cured, making challenge trials too dangerous to run ethically. Until now.
A Model Organism
The McShane lab’s solution is graceful in its simplicity: use BCG itself to test other vaccines.
“What you need in a challenge model is an organism that you can measure, because the outcome in a challenge model is pathogen loads,” McShane says. In a TB challenge, the question will be how many participants are infected, and how many bacteria are they infected with.
“So you’ve got to have a live organism to measure.”
TB is dangerous — but BCG fits the bill nicely. The key ingredient in BCG is a weakened form of Mycobacterium bovis, a close relative of TB that infects cows but does not cause disease in humans (similar to how the cowpox virus protects people against smallpox). The bacteria is already produced to high, human-safe standards to make the BCG vaccine, is cheap and readily available, and has a century’s worth of safety data.
All of which makes it, well, a model model organism.
A TB challenge trial using BCG would look something like this: you take a group of people who are vaccinated with your new vaccine candidate and a group of people who are not. You “challenge” them both with BCG and look for two end results.
Most obvious is the share of people who are infected. If you have zero infections in your vaccine group but everyone gets infected in the control group, your vaccine was 100% effective in the trial, making it a good candidate indeed. (“Infection” here means having detectable levels of the bacteria; wildtype M. bovis can make people sick, but the BCG version is weakened or “attenuated” to prevent it from causing disease.)
More subtly, you can compare how much BCG is detected in the vaccine group versus the control group.
The details of the experiment are a little tricky. There are two different ways to challenge volunteers with the bacteria.
You can inject BCG under the skin — this is how BCG is used to vaccinate kids against TB. Then, using a punch biopsy at the end of the challenge, researchers could measure how much BCG remains. If the vaccinated people clear more bacteria, more quickly, than the control group, the vaccine is likely working.
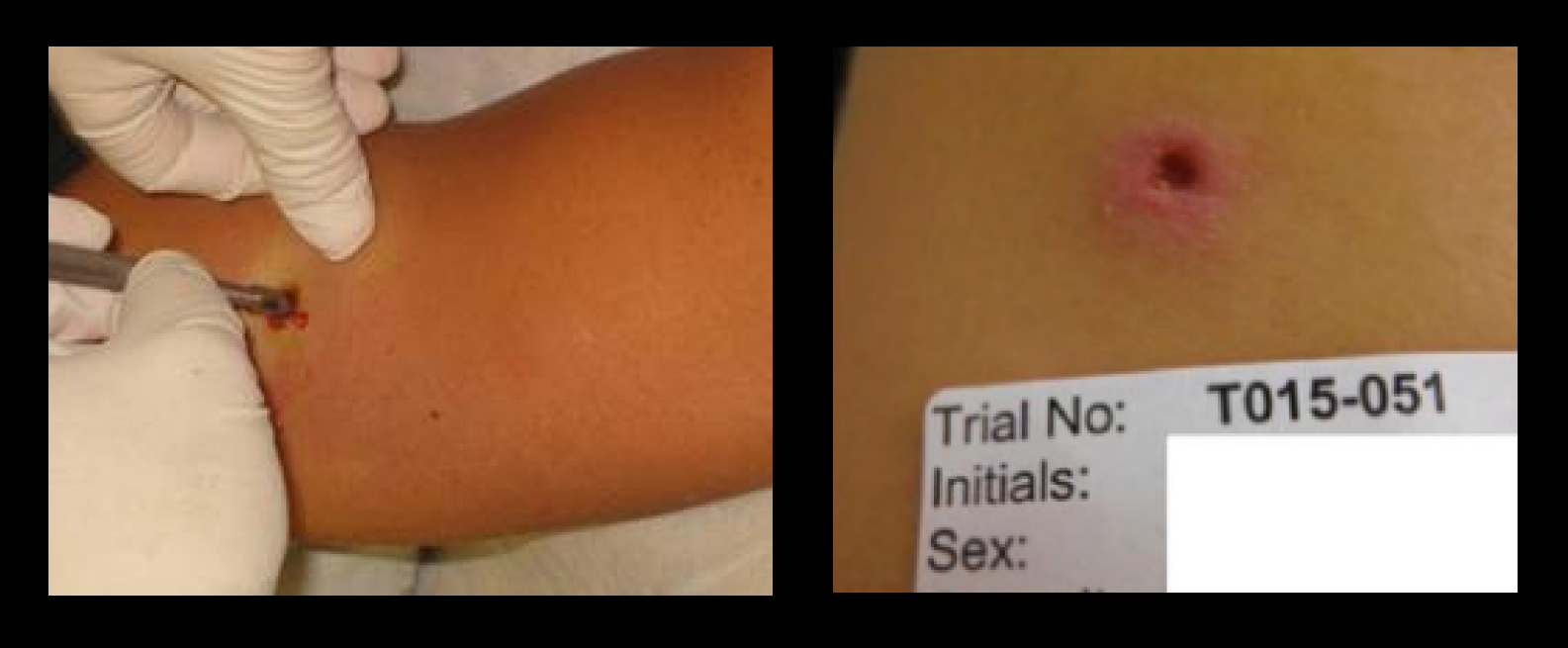
The second way to challenge people is breathing the BCG in via a nebulizer. This mimics how people catch TB in the wild, but it requires going into the lungs for measurement, a much trickier task. Either approach has its strengths and weaknesses, McShane says, and head-to-head studies will be needed to see which may be most beneficial for vaccine development.
“I think that there are limits to what it will tell us,” UW’s Casper says of the BCG model. “But at the same time, I think that it is incredibly exciting work, because we will learn things about how the body responds to mycobacteria in the lungs that we didn’t know previously.”
Modifying TB
There’s a few big questions still unsettled about the BCG method, Harvard’s Fortune says.
First, while BCG is closely related to TB, it is not TB. “There’s an inference that’s being made,” as Casper puts it, that efficacy against one will correlate with efficacy against the other.
Second, how easily can the bacteria be counted up? If it’s not simple to measure the infection, the trial won’t give clear results.
And finally, how well does the experiment mimic real-world TB infections? To do the trial, you’ll need to inject people with a sizable amount of BCG bacteria — so that measuring can be done — but is that comparable to TB’s ability to infect people with so few cells you could count them on one hand?
Fortune and colleagues are working on another way to make TB challenges safer, by modifying the TB bacteria itself.
To prevent TB from surviving antibiotics and the immune system and hiding in a challenge volunteer, Fortune’s lab is engineering TB bacteria with “kill switches” inside them. If you remember the Jurassic Park method, it’s something like that: engineering the bacteria to be dependent on a specific compound to live, which has to be taken during the course of the challenge. When the trial is over, the compound stops coming, and any remaining TB dies.
Such a method has to be safe, however, and the bar is high. Escape rates — the risk of bad mutations that could free the bacteria from their dependency — need to be on the order of 10–12 or 10-14. That means not one in a million million or one in a hundred million million bacteria can evolve an escape mutation.
“That’s hard,” Fortune says.
To help make the bacteria easier to measure, TB delivered under the skin could be engineered to glow green. Count the glow, and you’ve got an accurate measure of the amount of bacteria there.
Getting an accurate count in the lung is, of course, more difficult. A possible solution could be engineering “reporter systems” into the bacteria strain, which would release signals that could be measured in the breath, blood, or another easier to test area.
McShane considers the idea of engineered TB “exciting work,” but notes that more work is needed to prove it can work
“A Real Life-Changer”
Underpinning all of this is the tension at the heart of HCTs: do their benefits outweigh their risks?
Tuberculosis is very treatable. But the patients who contract TB in the developing nations where it is most common face multiple hurdles. Health centers may be many miles away, making them difficult and potentially costly to get to — sometimes making the choice either medicine or food, 1Day Africa’s Kafuko says.
“For these countries, for these people, for these communities, a tuberculosis vaccine that is efficacious, that is available, would be a real life changer.”
Zacharia Kafuko
“They start skipping,” Kafuko says. “And you know what happens when patients start skipping their [drug] courses.” If they skip for too long, they must begin again — and incomplete courses of antibiotics is a recipe for drug resistance.
In places where it still kills, TB is a scourge. Some of the communities it impacts are ready to be challenged to curb the threat; science may soon be ready to help them.
“For these countries, for these people, for these communities, a tuberculosis vaccine that is efficacious, that is available, would be a real life changer,” Kafuko says.
“It would literally translate into hundreds of thousands of lives being saved every year.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].