Senescent cells are typically associated with aging and health problems, but a new study adds to the growing evidence that “zombie” cells can actually be beneficial.
Senescent cells: Your body contains trillions of cells, and every day, some of them are dividing to create new cells, while others are dying and being cleared from the body to make room for their young replacements.
As we age, we start to accumulate senescent cells, or “zombie cells,” in our tissues. These cells are no longer capable of dividing, but they also haven’t been removed from the body. Instead, they linger, sending out chemical signals that trigger inflammation, which contributes to cancer, Alzheimer’s, and other diseases.
“From animals like Hydractinia, we can expand our understanding of aging and healing.”
Andy Baxevanis
The anti-hero: Recently, scientists have started to realize that senescent cells aren’t always problematic — they can actually help certain animals, such as zebrafish and mice, repair damaged tissue.
Now, a new study, published in Cell Reports, has detailed how the cells help a tiny sea creature called Hydractinia regenerate its entire body.
“From animals like Hydractinia, we can learn about how senescence can be beneficial and expand our understanding of aging and healing,” said study co-author Andy Baxevanis from the National Institutes of Health (NIH).
The study: A relative of jellyfish, Hydractinia species typically live on hermit crab shells and have regenerative abilities that outdo even the impressive axolotl. While that amphibian can regenerate its brain, Hydractinia can regenerate their entire bodies from just a fragment of tissue.
Past research has shown that Hydractinia has a reserve of stem cells in its tube-like body that it taps into when it needs to regenerate a body part, but when the NIH researchers removed the creature’s mouth — far from this stem cell reserve — a new body grew from the amputated bit.
This led the scientists to speculate that the animal was somehow capable of reverting cells near its mouth into stem cells.
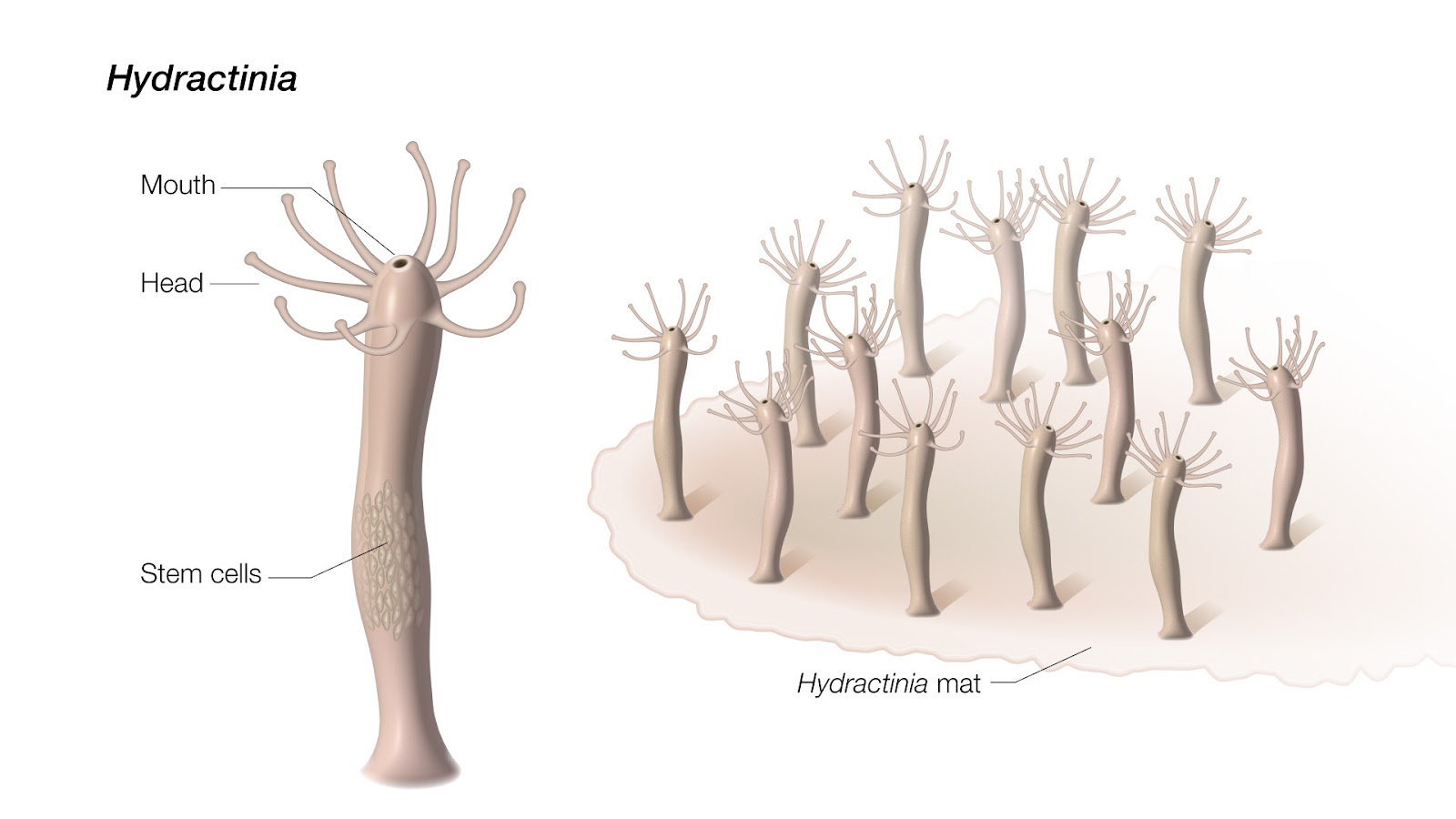
When they studied Hydractinia during the regeneration process, they detected signs of senescence in the cells near the injury. Based on that, they searched Hydractinia’s genome for genes similar to those linked to senescence in people.
They found three of those genes and discovered that at least one was expressed, or “turned on,” in cells near the site of the injury. When they blocked this gene, cells in the amputated mouth no longer showed signs of senescence — and Hydractinia couldn’t regenerate its missing body.
When the researchers let the process unfold naturally, they were surprised to see that the tiny organism would spit the senescent cells out of its mouth after it no longer needed them for regeneration.
The big picture: Because senescent cells are so commonly linked to aging and various health problems, many researchers are on the hunt for ways to clear them from our bodies. Studies like this one suggest there’s still a lot we don’t know about these zombie cells, though, so we may want to take a beat before trying to eliminate them.
“We still don’t understand how senescent cells trigger regeneration or how widespread this process is in the animal kingdom,” said Baxevanis. “Fortunately, by studying some of our most distant animal relatives, we can start to unravel some of the secrets of regeneration and aging — secrets that may ultimately advance the field of regenerative medicine and the study of age-related diseases as well.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].