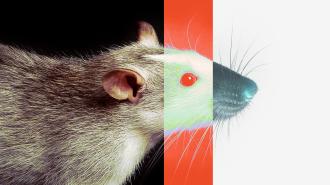
What do cuttlefish, scorpions, centipedes, snakes, and primates called slow lorises have in common? All evolved the relatively rare ability to produce venom — chemical toxins that kill or incapacitate other animals through bites or stings. And in a few thousands years, there’s a chance that scientists will add mice to that list.
That’s one of the takeaways of a new study that explored the evolutionary origins of oral venom systems in animals, which have until now remained little understood.
“Oral venom systems evolved multiple times in numerous vertebrates enabling the exploitation of unique predatory niches,” the researchers noted. “Yet how and when they evolved remains poorly understood. Up to now, most research on venom evolution has focused strictly on the toxins.”
In the new study, published in the journal PNAS, researchers instead focused on the gene-regulating networks associated with the production of venom in snakes. Because venom is a complex mixture of proteins, venom-producing animals have evolved a molecular system that’s capable of properly folding chains of amino acids in a highly specific way. Without this, animals wouldn’t be able to withstand the cellular stress caused by producing venom.
To better understand this process, the researchers examined venom glands of the Taiwan habu snake, a pit viper endemic to Asia. The goal was to identify genes that are strongly coexpressed with venom. The researchers identified 3,000 “housekeeping genes” (i.e., genes that are always turned “on”) that are associated with venom production, but primarily involved with protein folding and modification. They dubbed these nontoxic genes the “metavenom network.”
After identifying the metavenom network in snakes, the researchers searched for similar networks within the genomes of other animals: mice, dogs, and humans. The results showed that these animals also possess key structures of the metavenom network that’s found in snakes, suggesting mammals and snakes share a “common [gene] regulatory core” that traces back hundreds of millions of years to the species’ common ancestor.
The key phenotypic difference is that snakes use this shared regulatory core to produce venom, while most other animals use it to produce saliva.
“[T]his is the first real solid evidence for the theory that venom glands evolved from early salivary glands,” lead study author Agneesh Barua, a Ph.D. student at the Okinawa Institute of Science and Technology Graduate University (OIST), said in a press release. “And while snakes then went crazy, incorporating many different toxins into their venom and increasing the number of genes involved in producing venom, mammals like shrews produce simpler venom that has a high similarity to saliva.”
So, given that mammals and snakes share more evolutionary mechanisms than previously thought, could animals like mice someday evolve the ability to produce venom? Barua said it’s possible.
“There were experiments in the 1980s that showed that male mice produce compounds in their saliva that are highly toxic when injected into rats,” Barua said in the press release. “If under certain ecological conditions, mice that produce more toxic proteins in their saliva have better reproductive success, then in a few thousand years, we might encounter venomous mice.”
Overall, the study blurs “the line between venomous animals and their ancestors,” and highlights the fundamental similarities between animals that look and behave very differently upon first glance.
This article was reprinted with permission of Big Think, where it was originally published.