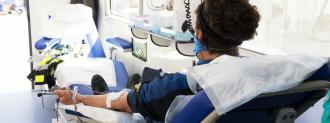
Convalescent plasma therapy — the use of blood plasma from a person who’s already recovered from an illness to treat someone still battling it — is currently one of the most promising treatments for the novel coronavirus SARS-CoV-2.
Now, to encourage more coronavirus survivors to donate plasma for COVID-19 trials and research, Microsoft is launching an online “Plasma Bot” to guide them through the donation process.
How (and Why) to Donate Plasma for COVID-19 Research
When a pathogen infects a person for the first time, their immune system produces antibodies that can help fight the virus or bacteria if it ever infects the person again.
It’s these antibodies that give plasma its ability to help people overcome a current infection.
Convalescent plasma has shown promise as a treatment for the coronavirus in several small trials, and now, hundreds of hospitals across the globe are participating in trials to test its efficacy on a larger scale.
The sooner recovered patients donate plasma, the sooner the alliance can start manufacturing a potential therapy.
Those trials need people to donate plasma for COVID-19 research, though, and they need them to do it as soon as possible — Microsoft’s head of research Peter Lee told CNBC the plasma is only useful from 21 days after the onset of coronavirus symptoms to about 56 days after.
To encourage donations, Microsoft created its Plasma Bot for the CoVIg-19 Plasma Alliance, an initiative to accelerate research into plasma as a coronavirus treatment.
The bot asks coronavirus survivors a number of questions to find out whether they could donate plasma for COVID-19 research, such as their current medications and how long they’ve been free of coronavirus symptoms.
If a person is eligible to donate, the Plasma Bot will provide information on the nearest plasma collection center.
Microsoft plans to launch the Plasma Bot on the Plasma Alliance’s website this weekend, as well as other places on the internet, in the hopes of reaching as many coronavirus survivors as possible.
Some of the people who decided to donate plasma for COVID-19 research through the bot will see their donations go directly to COVID-19 patients through a transfusion.
However, the Plasma Alliance has partnered with several of the collection centers to source plasma for its own research, which takes a different approach to convalescent plasma therapy.
“Time Is of the Essence”
Once the Plasma Alliance collects plasma from many donors, it plans to combine the donations and concentrate the antibodies into a liquid form.
It will then attempt to create a type of medicine out of the liquid called a polyclonal hyperimmune globulin (H-Ig) that it hopes to use to treat COVID-19 patients.
This medicine could have several advantages over direct transfusions of plasma from coronavirus survivors to current patients, according to the Plasma Alliance.
“Because H-Ig has a higher concentration of antibodies, it can be delivered in lower volumes, and therefore could take less time to administer to patients than plasma itself,” the group wrote on its website. “H-Ig also has a longer shelf life, which permits storage for an outbreak in the future.”
The Plasma Alliance also wrote that the process involved in manufacturing an H-Ig could reduce the potential risk of transmitting any kind of virus from a donor to a coronavirus patient, as well as allow researchers to set standards for antibody levels.
Before the group can put its theories to the test, though, it needs people to donate plasma for COVID-19 research.
“The sooner recovered COVID-19 patients donate convalescent plasma, the sooner the alliance may be able to start manufacturing a potential therapy and begin clinical trials,” Microsoft wrote in a blog post. “Time is of the essence.”