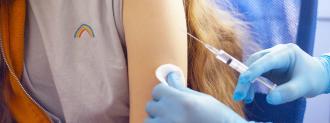
When the FDA authorized Pfizer’s COVID-19 vaccine in December, it did so with one caveat: people had to be at least 16 years old to get the shot.
That was because the original trial only tested the vaccine in people 16 and up — so we didn’t know how well it would work in younger people.
But that’s no longer the case.
Pfizer has just announced the results of a late-stage trial involving more than 2,000 adolescents between the ages of 12 and 15, and the vaccine appears to be 100% effective against symptomatic COVID-19 — even better than the 95% it announced for older adults.
Vaccinate the Youth
In general, younger people are somewhat less likely to catch COVID-19 than older people. They’re also less likely to have a severe case or die from the disease if they do catch it — but vaccinating them is still important.
Experts argue that in-person schooling is better for kids’ mental, physical, and social health, but many schools have transitioned to remote learning to prevent the spread of COVID-19 — vaccinating adolescents could make it less risky for those schools to reopen.
Moreover, even if young people are generally less susceptible to COVID-19, some have health conditions that put them in the high-risk category — the vaccine could be literally life-saving for them.
On a broader level, the U.S. simply needs to vaccinate kids if it wants to reach herd immunity. That goal requires around 80% of the population to be immune to COVID-19 — and more than 22% of Americans are under the age of 18.
Even if every adult got vaccinated, the virus may continue to spread through young people, potentially allowing it to break through into older adults (since the vaccine isn’t perfect) or evolve its way around the vaccine’s protection.
Back to the FDA
According to Pfizer’s announcement, only 18 of the adolescents in the trial contracted COVID-19 — and all of them were in the placebo group.
The vaccine was also well-tolerated, with side effects comparable to those experienced by people between the ages of 16 and 25.
The hope is to be vaccinating this age group before the start of the next school year.
Albert Bourla
The results of the trial have yet to be published in a scientific journal, but the FDA will independently re-analyze the data when Pfizer applies for authorization for the 12-15 age group.
If approved, the vaccine would become available for over 20 million adolescents in that age group, according to the U.S. Census.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Pfizer CEO Albert Bourla said in the announcement.
“We plan to submit these data to FDA as a proposed amendment to our Emergency Use Authorization in the coming weeks and to other regulators around the world, with the hope of starting to vaccinate this age group before the start of the next school year.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].