This article is an installment of Future Explored, a weekly guide to world-changing technology. You can get stories like this one straight to your inbox every Thursday morning by subscribing here.
Microscopic marine plants that pull CO2 from the air could play a huge role in helping stop climate change, according to proponents of a radical scheme to “fertilize” the world’s oceans with iron.
Deep sea storage: Slashing the amount of new greenhouse gasses we pump into the atmosphere is crucial to preventing the worst effects of climate change, but it won’t be enough by itself, according to climate experts — we also need to capture some of the carbon that’s already in the air.
One way to do this is by maximizing the carbon-capturing potential of Earth’s oceans — they already absorb 25% of human CO2 emissions, mainly due to the activity of phytoplankton.
These microscopic, single-celled plants grow near the ocean’s surface, taking in sunlight and CO2 to produce energy through photosynthesis. Some of that carbon is later trapped in the deep ocean when the phytoplankton die and sink.

Iron Age: Like other plants, phytoplankton need more than sun and CO2 to thrive — they also need nutrients, including iron, which is naturally scarce in some parts of the ocean.
We know from natural events in the past that increasing the amount of iron in these seas can dramatically increase the growth of phytoplankton. When iron-rich ash from volcanic eruptions has fallen on the ocean’s surface, it has triggered phytoplankton blooms large enough to see from space.
“Give me a half tanker of iron, and I will give you an ice age.”
John Martin
This knowledge led oceanographer John Martin to put forth something called the “iron hypothesis,” which suggests that “fertilizing” the ocean with iron could increase the amount of carbon-sucking phytoplankton — theoretically enough to cool the entire Earth.
“Give me a half tanker of iron, and I will give you an ice age,” he famously quipped during a lecture in 1988.
In 1993, shortly after Martin’s death, his colleagues at Moss Landing Marine Laboratories tested the hypothesis by increasing the concentration of iron over 64 square kilometers of the Pacific Ocean. They then observed the area for 10 days and saw the amount of plant biomass double.
“All biological indicators confirmed an increased rate of phytoplankton production in response to the addition of iron,” they wrote in a paper detailing the experiment.
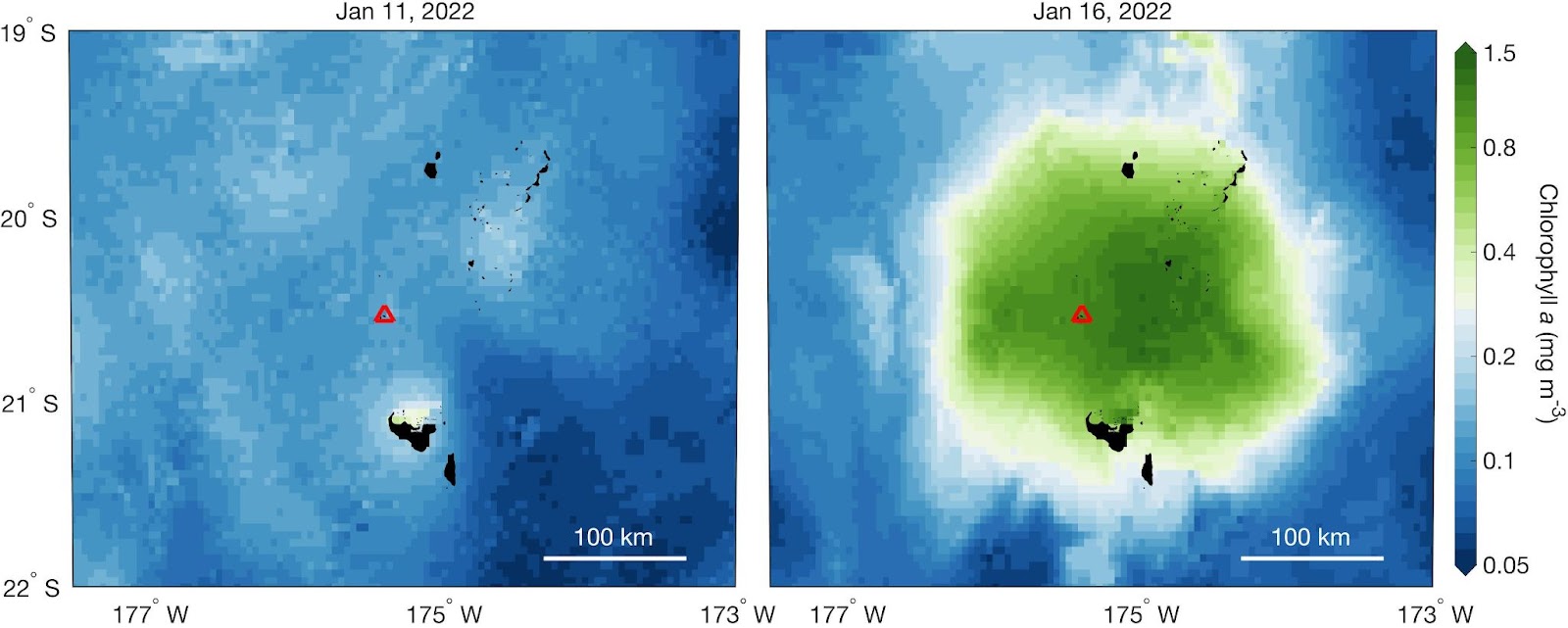
More than a dozen other ocean fertilization experiments have been conducted since then, but even though they do appear to cause a bloom of plankton, it’s still not clear whether the approach could actually help combat climate change.
In 2009, researchers from the Department of Energy’s Lawrence Berkeley National Laboratory tracked the impact of a major ocean fertilization experiment in the Southern Ocean between New Zealand and Antarctica by measuring carbon particles 800 meters below the surface of the water in the area for a year — and their findings were less than encouraging.
“Just adding iron to the ocean hasn’t been demonstrated as a good plan for storing atmospheric carbon,” said researcher Jim Bishop. “What counts is the carbon that reaches the deep sea, and a lot of the carbon tied up in plankton blooms appears not to sink very fast or very far.”
While researchers are still trying to figure out why that is, there are a number of theories, including ones centered on the feeding habits of creatures that live off phytoplankton and the presence of iron-binding organic compounds in ocean water.
Particle power: Some climate scientists aren’t ready to give up on the idea of ocean fertilization just yet, though.
In November 2022, an international team of researchers led by scientists at the University of Leeds and the DOE’s Pacific Northwest National Laboratory published a study suggesting that changing how we deliver the fertilizer might be the key to getting it to work.
Instead of simply adding iron sulfate to the ocean’s surface, these researchers propose we engineer nanoparticles of iron with characteristics that help overcome issues currently preventing ocean fertilization from living up to its potential.
We could coat the nanoparticles in polymers that make them buoyant, for example, keeping them near the surface to enhance the uptake by phytoplankton. Or give them light-absorbing properties that help the phytoplankton better absorb sunlight for photosynthesis.
We could even lace the nanoparticles with things other than iron — including silica in them, for example, would increase the density of phytoplankton that intake the particles, and a mere 1% increase in density can be enough to double their sinking speed, the researchers write.
“Examining all our options, including using the oceans as a CO2 sink, gives us the best chance of cooling the planet.”
Michael Hochella
While the researchers acknowledge that engineered nanoparticles would cost significantly more to produce than the iron sulfate typically used for ocean fertilization experiments, they would also be significantly more effective.
“Our study shows that the use of several types of engineered nanoparticles in ocean fertilization can be promising in terms of costs and carbon dioxide emissions during production and delivery processes,” said researcher Peyman Babakhani.
The big picture: While the recent DOE-backed study illuminates a promising path forward for ocean fertilization, adding enough iron nanoparticles to the oceans to affect the climate would be a huge undertaking, and much more research is needed to determine how it might affect ocean ecosystems.
“Large-scale fertilization could have unintended and difficult-to-predict impacts not only locally, but also at great distances in space and time,” according to researchers with the Deep-Ocean Stewardship Initiative.
As with other ideas for geoengineering our way out of climate change, there are also issues of public acceptance and government regulation to consider.
For now, there are other, less-risky ways to sequester atmospheric carbon — we can plant more trees, make farm soil more carbon-hungry, and deploy machines that trap carbon in chemical filters — but proponents of ocean fertilization urge others to study the option so that we’ll know the safest, most effective way to deploy it if drastic measures are required in the future.
“At this point, time is of the essence,” said Michael Hochella, an author of the recent study. “To combat rising temperatures, we must decrease CO2 levels on a global scale. Examining all our options, including using the oceans as a CO2 sink, gives us the best chance of cooling the planet.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].