Most of us only have to deal with dry eyes if we stare at a computer screen for too long (or consume too much of the devil’s lettuce).
For the estimated 5% of adults who suffer from dry eye disease, though, dry eyes are a daily struggle — and not only is it painful, in severe cases, it can even lead to blindness.
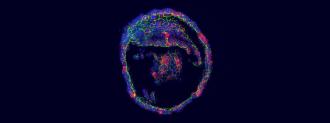
Now, Dutch researchers have grown tear gland organoids in the lab, potentially ushering in new treatments — or even a cure — for dry eye disease.
Dry Those Eyes
Dry eye disease is usually linked to some problem with the tear glands that are responsible for keeping the surface of the eye lubricated.
Those glands are situated right above the eyeball and behind the bony eye socket. That makes it difficult to take biopsies of them (not to mention extremely uncomfortable), which limits research on new treatments for dry eye disease.
Organoids — lab-grown clumps of cells that mimic the function of human organs — could help alleviate this research hurdle.
Scientists have already figured out how to grow heart, brain, and other organoids from stem cells in the lab, and while the mini models are nowhere near as complex as their full-sized counterparts, they do contain the same cell types found in those organs.
That makes them tremendously useful for research — scientists can use the tiny models to study organ development, disease progression, or new treatments.
Tear Gland Organoids
To create their tear gland organoids, researchers at the Hubrecht Institute and the UMC Utrecht in the Netherlands started with adult stem cells.
They coaxed those cells into turning into functional ductal cells, which are found in the tear gland.
If there had been a little duct, there would have been droplets.
Hans Clevers
The hardest part, according to researcher Marie Bannier-Hélaouët, was getting the cells to cry — to produce actual tear fluid.
“The challenge was to get the organoids to cry, as this is a hallmark of the (tear) gland,” she said in a press release. “We had to modify the cocktail of factors the organoids are grown in so that they would become the mature cells that we have in our tear glands and that are capable of crying.”
Finally, when they dropped a tear-inducing chemical on their organoids, the cells swelled up with tear fluid.
“If there had been a little duct, there would have been droplets,” researcher Hans Clevers told Nature.
What’s Next?
The researchers aren’t done developing their tear gland organoids.
They now want to figure out how to grow acinar cells — the other type of cell found in tear glands — in the hopes of eventually growing whole tear glands in the lab
Not only would those be even more useful for treatment research than the current tear gland organoids, they’d have the potential to cure dry eye disease outright — doctors might one day be able to grow replacement glands from patients’ own stem cells.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].